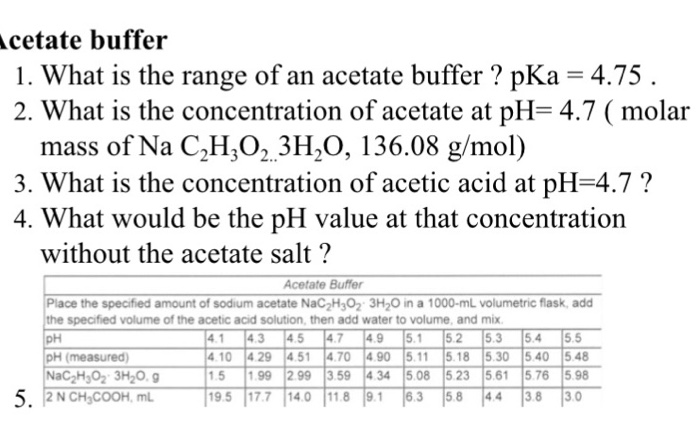
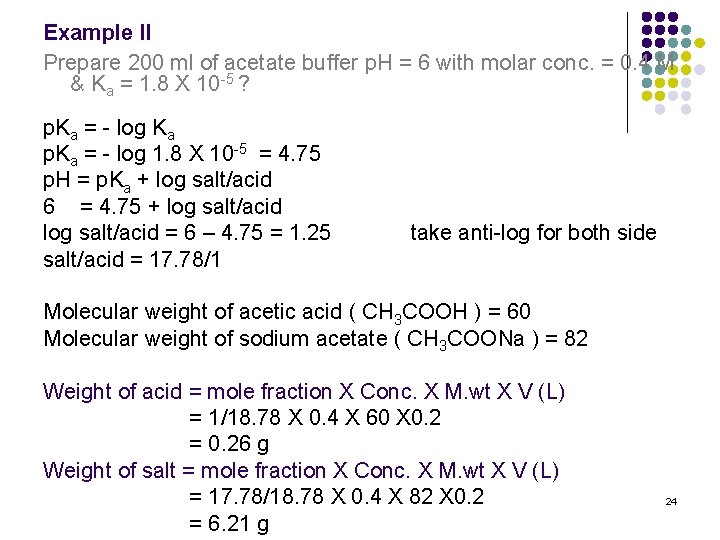
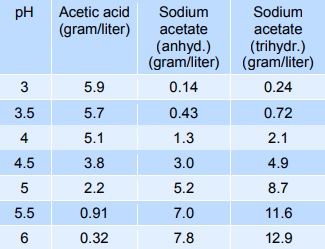
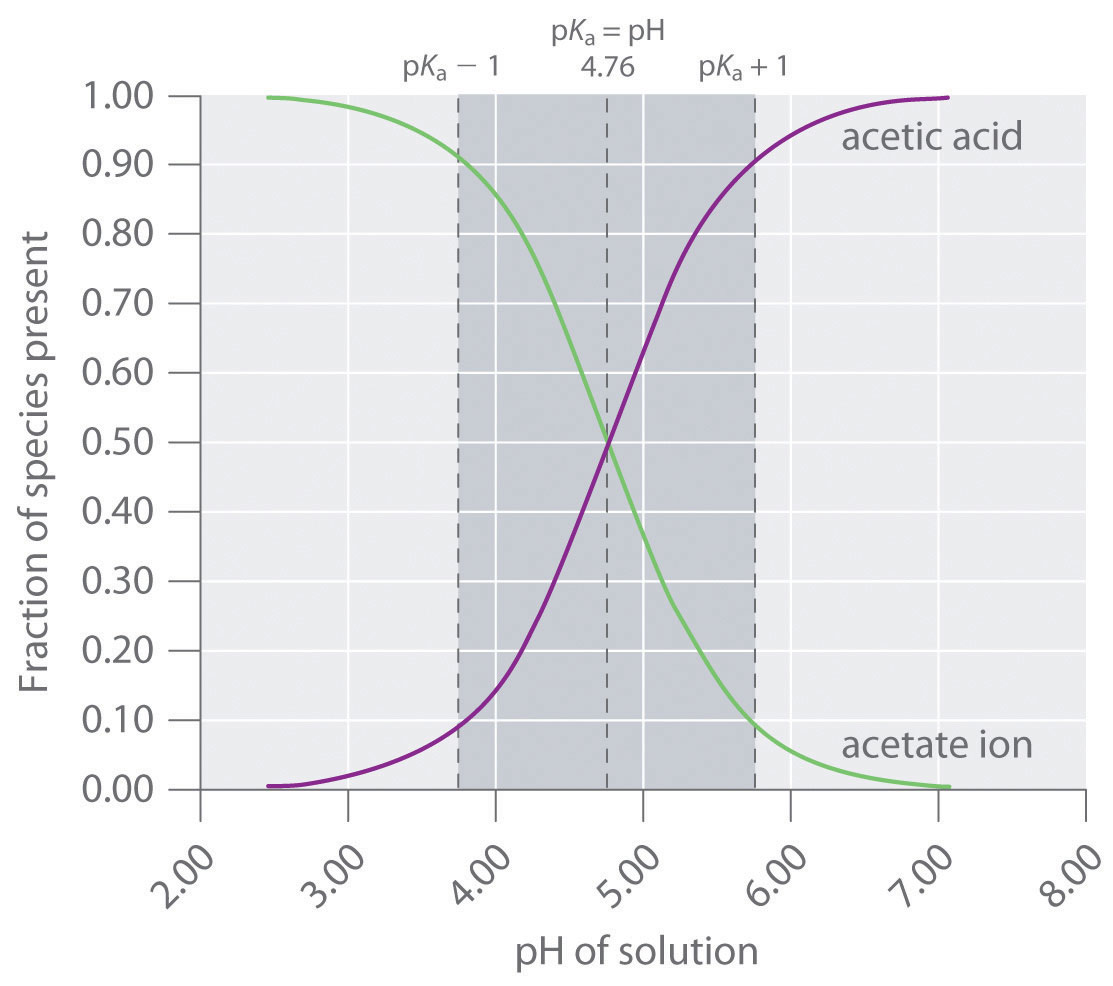
Effect of pH (in Na-acetate buffer, 50 mM, at pH 4.0-6.0 and in sodium... | Download Scientific Diagram
Compare 1 L of acetate buffer solution (0.50 mol of acetic acid and 0.50 mol sodium acetate) to 1 L of HCl solution Similarities

Sodium acetate buffer 5.2 +/- 0.1 25°C, for molecular biology, 3M, 0.2 μm filtered | 126-96-5 | Sigma-Aldrich

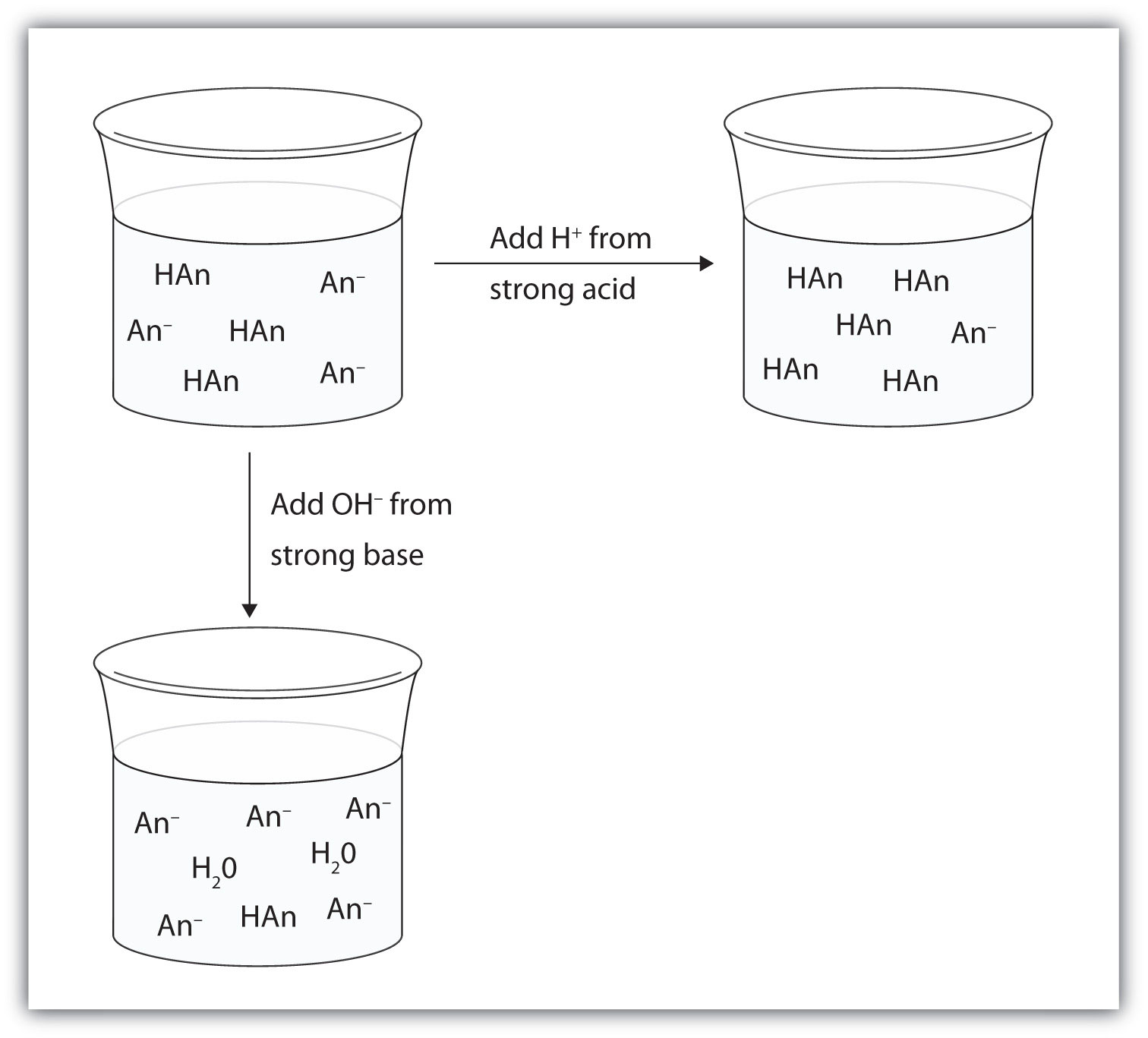
SCH 4 U 1. What are buffers? Buffers are mixtures of conjugate acid- base pairs that allow a solution to resist changes in pH when acids and/or bases. - ppt download

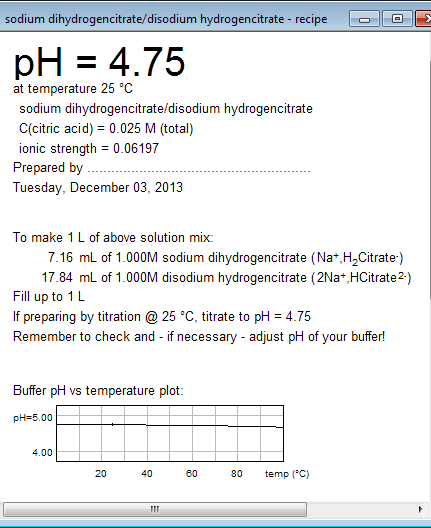
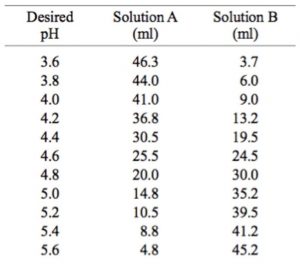


![Sodium Acetate, pH 5.2 [3M] Sodium Acetate, pH 5.2 [3M]](https://www.gbiosciences.com/image/cache/Sodium_acetate-500x500.jpg)